
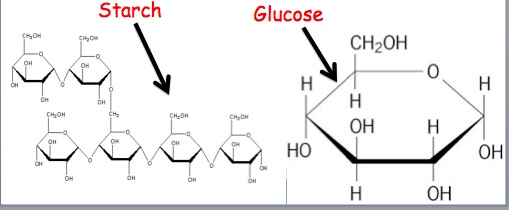
Polyethene (a mass-made plastic used in packaging) and Nylon Fibers are two examples of commercially created polymers that we utilize daily (commonly used in our clothes, ropes, etc.) These are manufactured commercially for human consumption. Synthetic polymers are polymers that can be created or synthesized in a lab by humans. Proteins (which have the same structure in humans and animals), Cellulose and Starch (which are present in plants), and Rubber are all common examples (which are harvested from the latex of a plant). Natural polymers arise naturally and can be found in natural sources such as plants and animals. The easiest method to categorize polymers is by their origin. Let's look at the first classification of polymers based on their source of origin. Glyptal is actually made up of monomers ethylene glycol and phthalic acid.īakelite can also be called poly-oxy-benzyl-methyl englycol anhydride, which is a plastic that is made up of monomers phenol and aldehyde. The urea-formaldehyde resin is not transparent in nature the plastic is obtained by heating formaldehyde and urea. Polyvinyl chloride (PVC) is said to be a plastic polymer that is made of monomer vinyl chloride.
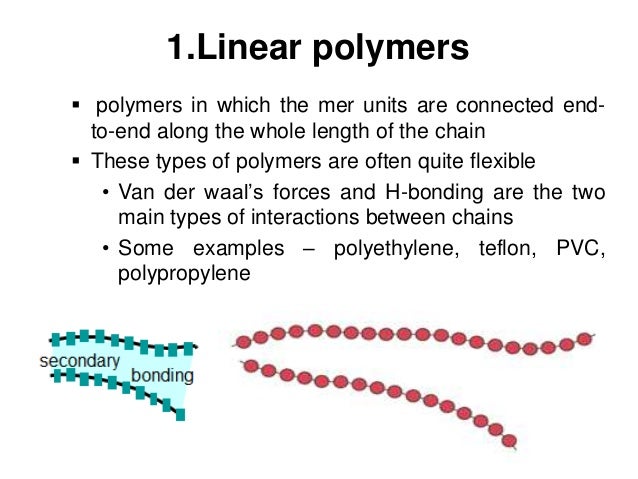
There are also a few polymers that, alternatively of having a carbon backbone, have phosphorus or silicon backbone. Polycarbonates and Polyesters contain an oxygen atom in the backbone. Nylons contain nitrogen atoms in the repeated unit of the backbone. Other commonly produced polymers have backbones that have elements other than carbon. The Teflon has fluorine attached to all the carbon backbone. Polyvinyl chloride (PVC) has a chlorine atom attached to all the carbon backbone. Polyethylene, polybutylene, polystyrene, polypropylene, and polymethyl pentene are examples of these polymers. There are polymers that have only carbon and hydrogen atoms. We can attach one or more other atoms to each carbon atom in the backbone chain. The polymers are exactly made of carbon atoms bonded together, one to the next, into large chains that are called the backbone of the polymer. Many general classes of polymers are formed of hydrocarbons, hydrogen, and compounds of carbon. Nylon was invented in the year 1935 while trying synthetic spider silk. The first produced polymeric fiber was Rayon, from cellulose, in the year 1910. The very first synthetic manufactured plastic was Bakelite, in the year 1909 for telephone casing and electrical components. We use rubber tree latex and cellulose as raw materials to make fabricated polymeric rubber and plastics. A Starch can be a polymer as it has cellulose in wood. Hair, Spider silk, and horn are the protein polymers. The ultimate natural polymers are deoxyribonucleic acid i.e., DNA, and ribonucleic acid i.e., RNA that explain life. The Cups, Plastic bottles, Films, and fibers are Thermoplastic plastics. These chains are Thermoplastic polymers and can also be called Linear polymers.

These manufactured polymers can also be a 1-dimensional chain that can be melted. Epoxy resins which are used in 2-part adhesives are thermoset plastics.
Such networks are called Thermoset polymers. The polymers that can be manufactured can be 3-dimensional (3D) systems that do not go once formed. Polymers can occur naturally and can be made to serve particular needs. Linking infinite strips of construction paper together to create paper garlands or attached together hundreds of paper clips to make chains, or stringing beads helps you to visualize the polymers. This forms a chain, many links or “-mers” are chemically attached or polymerized together. The unit which is repeating is often made of hydrogen and carbon and sometimes nitrogen, oxygen, fluorine, sulfur, chlorine, silicon, and phosphorus. Each unit that repeats is the “-Mer” or has a fundamental unit with “polymer” meaning multiple repeating units. A polymer can be a 3-dimensional (3D) network Imagine of a repeating unit joined together left and right, back and front, up and down or it is a 2-dimensional (2D) network Imagine of the repeating units linked together right, left, down, and up in a sheet or a 1-dimensional (1D) network Imagine of a repeating unit-linked right and left in a chain. The simplest way to understand the term polymer is a beneficial chemical made of many repeating units.


 0 kommentar(er)
0 kommentar(er)
